Complex crystalline alloys are important in many applications as materials with specific mechanical and electrical properties and melting/ solidification behaviour. Yet, their solidification and mechanics are difficult to study directly at the atomic scale. Recently, we realized analogues of these materials using colloidal particles, i.e. micron-size particles that can be engineered with exquisite control over size, shape, and mutual attractions. These colloidal building blocks can be made with finely tunable interactions so that they form complex structures just like atoms do, but on a much larger scale, making them directly observable in real-space and time. Besides being models for atoms, the particles also serve as new building blocks for novel nano- and micro structured materials used in photonics and optoelectronics.
The goal of this project is to design crystalline alloys from two types of particles, A and B, and investigate their formation. Just like in atomic alloys, mixing particles A and B of certain size ratio, and relative attraction (interaction energies uAA, uBB, and uAB) gives rise to specific crystal structures. We have a large collection of A and B particles available with specific size ratio and relative attraction that we can vary with external control. This allows us to “freeze” the particles from a colloidal liquid into complex crystal structures, and by investigating the process, obtain unique insight into the crystallization kinetics of these complex crystals. Because the particle sizes are of the order of a micrometre, we can image them in three dimensions using confocal microscopy. We can even follow the trajectories of the individual particle s in three dimensions to elucidate kinetic pathways in the formation of the structures. The movie below shows a crystalline microstructure of a strongly attractive particle A (bright) with a less attractive particle B (faint blue), “quenched” in a complex colloidal microstructure. Such direct-space observation provides unique insight into basic mechanism of (atomic) assembly.
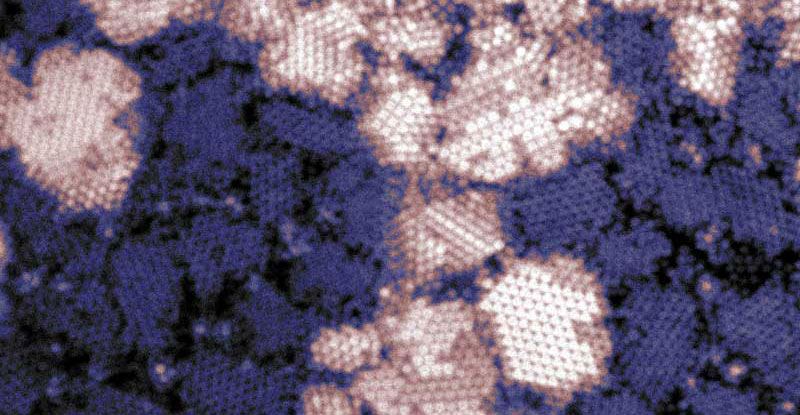
The figure above shows a microscope image of the particle-scale structure of a colloidal polycrystal assembled from type A (bright) and type B particles (blue). Particles A and B separate due to their largely different attraction. This is shown when we decrease the attraction to melt the crystals, see movie below: Crystals of particles B melt first, while particles A are still crystallized.