Check out our recent publication in Nature comm.
We use gels everyday. We hold our hairstyle together with hair gel. We eat gelatine, jelly, and gummy bears. Gels are soft, yet they resist small stresses. Unlike conventional solids, gels form with much fewer particles, yet they still fill a large volume. Gels are made of a connected network of particles suspended in a liquid; though the by far largest part of gel is solvent, it is still able to stand on its own!
We investigate how gels form and their rigidity arises. Using colloidal particles interacting with critical Casimir forces, we monitor the build-up of the space-spanning network upon increasing the attractive strength.
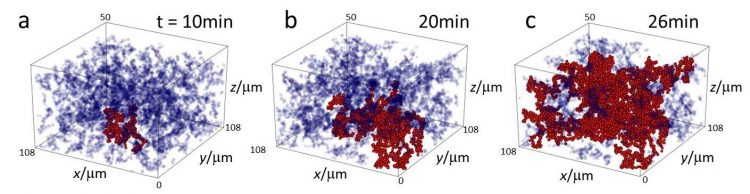
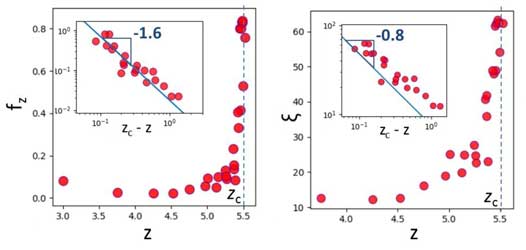
Interestingly, the growth displays a power law. The size of the largest cluster diverges upon approaching the gelation point. Since the growth process occurs in an out-of-equilibrium process (detailed balance is broken as the attachment rate is much higher than the detachment rate), these observations suggest that the gelation is a second order non-equilibrium phase transition.