Colloidal particles offer fascinating insight into the statistical mechanics and assembly behaviour of atoms. The particles, about a micrometer in size, have thermal energy and attractive/repulsive interactions similar to atoms, making them form phases very similar to their atomic counterpart. Yet, due to their larger size, they can be easily observed by optical microscopy. Besides being atomic models, these particles serve as building blocks for the assembly of new micro- and nanoscale materials that are used, e.g. in photonics and optoelectronics.
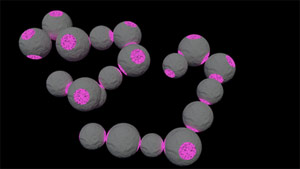
We bind “patchy” colloidal particles into colloidal molecules using critical Casimir interactions. The attractive critical Casimir force arises only between the patches, from the confinement of critical solvent fluctuations between them. The movie below shows a pair of tetrapatch particles (particles with four patches in tetragonal coordination) clearly bonded via one of their patches (bright). These tetrapatch particles form structures known from molecular carbon (in sp3 hybradization), such as alkanes and more complex molecules, see figure below. Because we can follow these colloidal molecules directly in real space and time, we obtain unique insight into their atomic counterpart, dynamics and transition states. We analyse their vibration spectrum, conformations and relaxation, and how this depends on the patch attraction, which we can vary continuously.

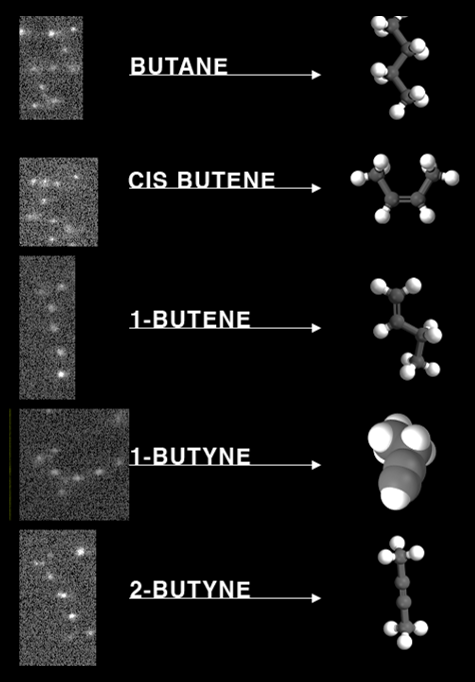
Colloids with directed interactions assemble into colloidal molecules, with structures mimicking that of atomic molecules. The figure shows colloidal molecules assembled from di- and tetrapatch colloidal particles. Optical microscopy images are shown on the left, and their molecular analogues on the right. These particles form molecular compounds known from organic chemistry, and because of their size can be directly observed at the particle scale by microscopy. Furthermore, their three-dimensional structure and dynamics is conveniently observed. As the colloidal molecule is in a highly thermally excited state, where quantum mechanical states become quasi continuous, it corresponds to the classical limit of its atomic counterpart, and as such presents a good model of the actual molecule.